Contact
On our page you will find a multitude of information that may be of your interest. If you have any questions, do not hesitate to contact our team:
Articles
and news

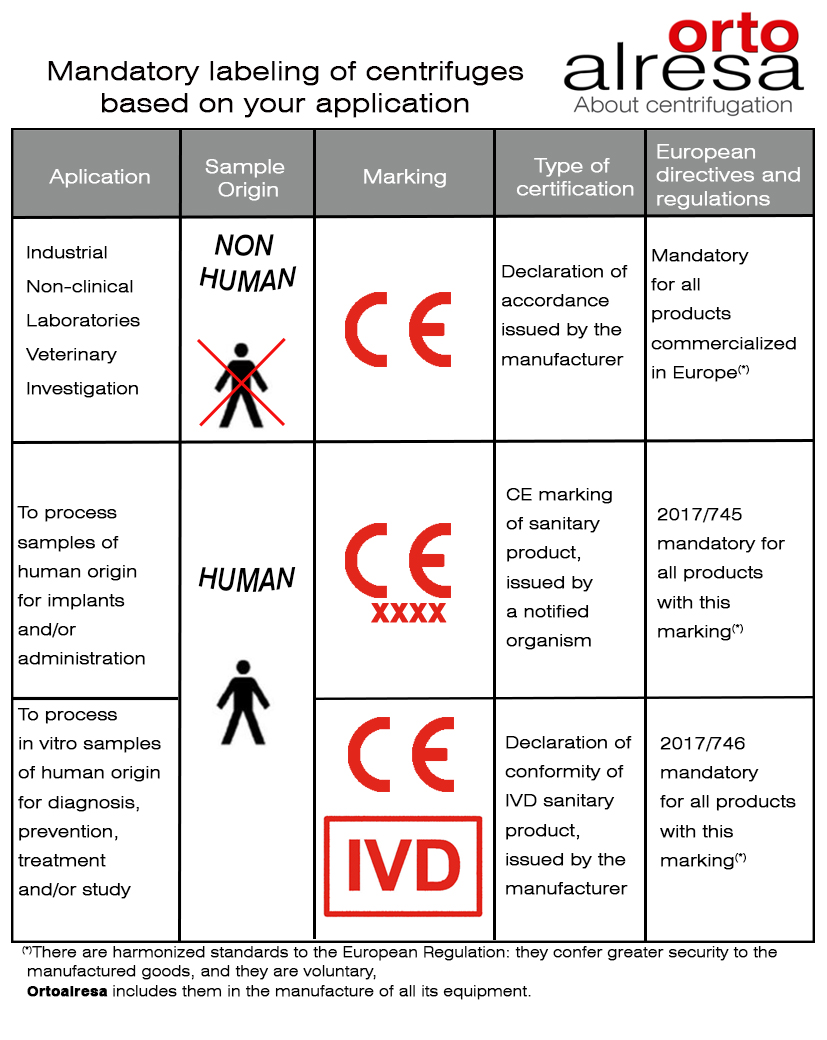
For some time now, centrifuges, like other laboratory equipment, must be accompanied by specific certificates and labels depending on the regulations and their use in the laboratory.
Given the confusion sometimes generated by compliance with the regulations and directives that define this type of labelling, from Ortoalresa, we want to offer some basic guidelines for the appropriate choice of centrifuges.
This brings us to the first basic question…
CE marking of conformity. It is mandatory to be able to market in Europe indicating that the product is manufactured in compliance with the mandatory legislation on European essential requirements. It is found both on the characteristics label of the centrifuge and in the declaration of conformity issued by the manufacturer and accompanying the equipment.
Some applications that require only this type of marking:
Regarding the selection of laboratory centrifuges, we must take into account the application and the requirements generated from it, since they may lead us to require other types of certifications, such as the CE marking of medical devices and the CE marking – IVD (in vitro diagnostic medical device).
This type of labelling and certifications is needed only for applications related to samples from human beings.
See/Download
By definition in the Regulations and as a general concept:
As a common element to determine what type of marking the centrifuges should have, we have the application to which the equipment is going to be used. Therefore, the user must be the one who determines said application, in order to know what type of certification he will need.
Ortoalresa offers a wide range of centrifuges for all types of applications, with the necessary certifications to meet the requirements of each laboratory.
If you wish to receive our latest news periodically, do not hesitate to subscribe to our Newsletter.
* Required fields. These fields must be completed to submit the form. Thank you
I have read and accept the privacy policy of the Company.
I agree to receive your commercial communications